ISARIC4C Comprehensive Clinical Characterisation Collaboration
ISARIC4C is an open, inclusive UK-wide collaboration of doctors and scientists committed to answering urgent questions about emerging infections and public health threats quickly, openly, and for the benefit of all.
ISARIC4C is the link between scientists, public health agencies, and NHS teams across the UK.
Since 2012 we have been preparing for outbreaks worldwide. International Severe Acute Respiratory Infection Consortium Clinical Characterisation Protocol.
ISARIC4C was at the core of the UK’s response to COVID-19
“…it has captured a large proportion of UK hospital cases and it has proven extremely useful to both SAGE and SPI-M. It has, for example, been vital in identifying risk factors associated with poor outcomes as well as well as early signs of the prevalence of hospital-acquired infection.”
Sir Patrick Vallance, UK Government Chief Scientific Adviser and Head of the Government Science and Engineering Profession, written evidence to Parliament (24 July 2020)
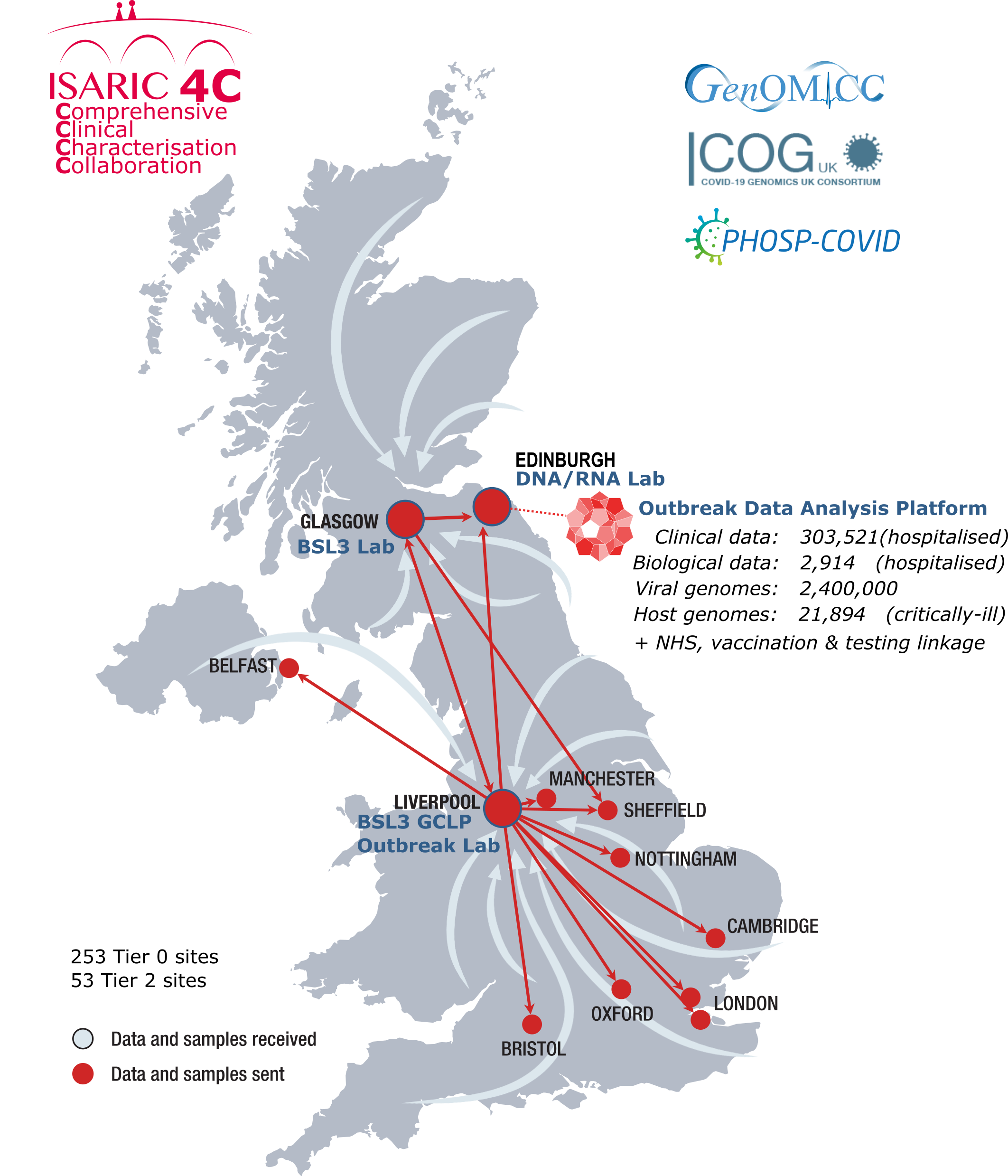
Recruitment
ISARIC4C is currently actively recruiting cases in the UK with:
- unexplained acute hepatitis
- monkeypox
Analysis Platform
The ISARIC4C study has created an open-access integrated analysis platform for linked clincal data from across the NHS for a range of studies, including ISARIC4C, GenOMICC, PHOSP, COG-UK and UK-CIC.
Funding
ISARIC4C is funded by UK Research and Inovation (UKRI) and The National Institute For Health Research (NIHR). We share data and samples to get answers as fast as possible. Any investigators with the ability to contribute can access our data and samples. The ISARIC4C study provides a foundation for other studies, such as clinical trials of new treatments, to help better understand the best way to use interventions.
Outputs
SARS-CoV-2 antibody responses
ISARIC4C have, in this study shown discernible differences in the humoral immune responses betwee...
Adeno-associated virus 2 infection in children with non-A-E hepatitis
ISARIC4C researchers found the link between adeno-associated virus 2 (AAV2) and acute hepatitis o...
Antibody responses in dried blood spots
There is good agreement between the neutralising antibody (nAb) responses measured in eluted DBS ...
Genetic Variants underlying Critical COVID-19
ISARIC4C researchers and the GenOMICC team have previously shown that genetics can help us to cho...
Pediatric COVID-19 Severity
Measurement of COVID-19 severity in children is key to determine the implications associated with...
Recovery from COVID-19 critical illness
Fatigue may be less severe in patients who have recovered from Covid-19 than after other illness....
Genomic investigations of unexplained acute hepatitis in children
ISARIC4C researchers worked closely with researchers led by Prof Judith Breuer at Great Ormond St...
Whole-genome sequencing reveals host factors underlying critical COVID-19
This research article outlines key findings from the GenOMICC study, using whole-genome sequencin...

